Clean rooms are highly controlled environments where the main objective is to limit the presence of airborne particles. Their design and operation are regulated by the ISO 14644-1 standard, which is the basis for all clean room classifications according to the level of air purity. Additionally, GMP regulations complement these standards in pharmaceutical environments, requiring optimal conditions for the manufacturing of products in a sterile room.

ISO 14644: the international reference
The ISO 14644 defines nine classes of clean rooms, with ISO class 1 being the most stringent, having a maximum of 10 particles ≥0.1 µm/m³. To achieve this level, all elements of the environment must be designed to not generate or accumulate particles. The lighting fixtures, in particular, must be completely sealed, flicker-free, and suited for laminar airflow.
Classification of clean rooms – ISO 14644-1
|
ISO Class |
≥0.1 µm |
≥0.2 µm |
≥0.3 µm |
≥0.5 µm |
≥1 µm |
≥5 µm |
|
ISO 1 |
10 |
– |
– |
– |
– |
– |
|
ISO 2 |
100 |
24 |
10 |
– |
– |
– |
|
ISO 3 |
1 000 |
237 |
102 |
35 |
– |
– |
|
ISO 4 |
10 000 |
2 370 |
1 020 |
352 |
83 |
– |
|
ISO 5 |
100 000 |
23 700 |
10 200 |
3 520 |
832 |
– |
|
ISO 6 |
1 000 000 |
237 000 |
102 000 |
35 200 |
8 320 |
293 |
|
ISO 7 |
– |
– |
– |
352 000 |
83 200 |
2 930 |
|
ISO 8 |
– |
– |
– |
3 520 000 |
832 000 |
29 300 |
|
ISO 9 |
– |
– |
– |
35 200 000 |
8 320 000 |
293 000 |
GMP regulations and manufacturing in a sterile room
In addition to the ISO clean room standards, compliance with GMPs (Good Manufacturing Practices) is mandatory in the production of sterile medications. This GMP regulation classifies rooms by grades (A, B, C, and D), with grade A being the most stringent and comparable to an ISO 5. This framework regulates both air quality and thermal, pressure, and flow conditions, which are essential to prevent any risk of contamination.
In a sterile room under GMP regulations:
- The lighting must be uniform, without creating shadows or areas of particle accumulation.
- Total control of humidity (between 40-60%), temperature (with minimal tolerances), and static electricity is required.
- The installation must be connected to an exclusive ground network and use non-particulate materials.
Additional considerations
Key factors to ensure the proper functioning of a clean room:
- Personal contamination: users themselves can be a primary source of particles, so appropriate clothing is required.
- Types of airflow: laminar flow (removes particles consistently) or turbulent flow (dilutes contaminants).
- Closed circuit systems: especially useful when handling sensitive or toxic substances.
Zone distribution and laminar flow in clean rooms
In sectors such as pharmaceuticals, food, or biotechnology, clean rooms must have clearly defined areas and a laminar flow system that ensures the removal of contaminants.
Two key elements in this structure are:
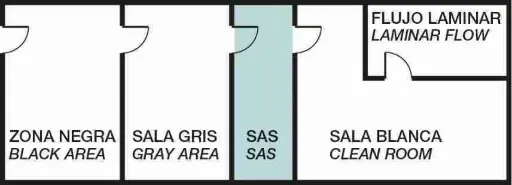
SAS (Safety Access System):
Pass-through chambers that allow the transfer of materials or people between areas with different cleanliness levels without compromising sterility.
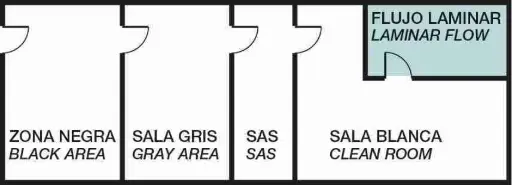
Laminar flow enclosures:
Spaces where air circulates in a single direction, at a constant speed, passing through HEPA filters. This keeps the critical work area free of particles (≤ 0.1 µm).

International Organization for Standardization (ISO). (2015). ISO 14644-1: Cleanrooms and associated controlled environments – Part 1: Classification of air cleanliness by particle concentration. Recuperado de https://www.iso.org/standard/53394.html